Cellular and Molecular Imaging
CELLULAR AND MOLECULAR IMAGING

VolumeScope is a Serial Block-face Imaging (SBFI) instrument for automated acquisition of large volumes. It integrates with Thermo Fisher Scientific SEM platform. The key enabling technologies, hardware, and software include:
- Novel Field Emission Electron Optics for both high resolution and exceptional contrast, meeting all imaging needs at low beam currents.
- Fully-integrated, compact, stage-mounted ultra-microtome for in-situ sectioning.
- Enables direct correlation of images from any light microscope covering large volumes.
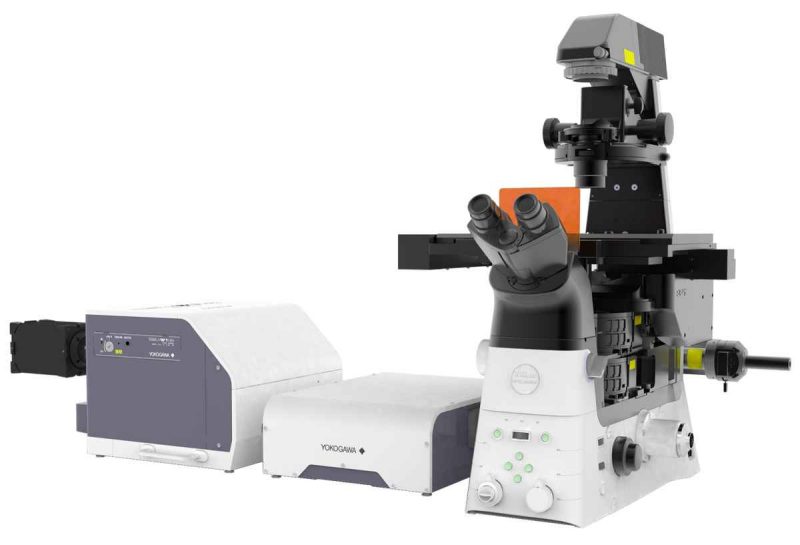
The Nikon SoRa (super resolution by optical reassignment) provides conventional confocal scanning, SoRa at 2.5X and 4X resolution, and TIRF imaging captured by 3 hamamatsu cameras. It allows a 1.4X improvement in lateral resolution beyond the optical diffraction limit.
The Nikon SoRa can collect super resolution fluorescence images as well as conventional confocal, TIRF, wide field, and transmitted light images (bright field and DIC). The scope has 10x, 20x, 20X water, 25X silicon, 40X water, 60x TIRF oil, and 60x oil objectives. A water adjustment system allows for the proper water to be applied throughout the experiment while a stage-top incubator adjusts temperature and CO2. The iLas2 and photostimulation with galvo scanner provides 360 degree TIRF imaging.
Lasers: LunF-XL solid state laser combiner with 405, 445, 488, 514, 561, 594 and 640nm imaging lines and 405, 473 stimulation lines
Filters: DAPI, GFP, RFP, Cy5
Cameras: 3 Hamamatsu Back-Thinned Orca Fusion CMOS cameras
Software: NIS Elements, 2D/3D deconvolution, iLas2
Computer: HP Z8 microscope computer and separate workstation for analysis
The Nikon SoRa Workstation provides software to analyze images from the Nikon SoRa. The workstation operates with NIS-Elements to provide user defined protocols, 3D tracking and analysis, constrained iterative deconvolution in 2D and 3D with point spread function support for ASC and ISM modes, and artificial intelligence image processing suite.
Thermo Scientific Amira Software is a powerful toolbox that enables cell biologists to visualize, process, and analyze complex biological data in 3D. With its advanced image processing, visualization, and analysis tools, Amira software can help you gain insights into the structure and function of cells and tissues that would be impossible to see with 2D images alone.

Includes inverted stage (4 laser lines – 405 / 488 / 555 / 647) equipped with a live cell imaging incubator and an automated stage for automatic scanning and tiling of large images.
Uses a Zeiss AXIOExaminer inverted microscope stand with transmitted (HAL), UV (HBO) and laser illumination sources. Can collect transmitted light images (bright field and DIC) as well as conventional and confocal fluorescence images. Has 10x, 20x, 40x (oil), and 63x (oil) objectives and a fully automated platform, including a live cell imaging incubator and automatic scanning and tiling of large images. Provides Z-series, 3D/rotatable reconstruction, time-lapse analysis for following cellular events in live samples, and co-localization of multiple proteins and multicolor imaging of fixed samples with a wide array of imaging applications including protein localization analysis, cytoskeletal studies, live cell imaging and cell cycling studies.
Fluorescence Filters: DAPI, DsRed, GFP, mPlum
Lasers: 488 / 555 / 594 / 633
Software: ZEN software to control the microscope, scanning, laser module, tools and the image acquisition and processing
Computer: HP Z820 workstation with the Windows 7 (64 bit) operating system

The FEI Tecnai G2 Spirit Twin Transmission Electron Microscopy (EM) is a multi-purpose- high resolution instrument. The cooled column and LaB6 emitter povide stable low-dose observation of beam sensitive samples while the 20-120kV capacity allows for imaging biological and material science samples. Features include:
- An FEI Tecnai G2 Spirit Bio-Twin TEM operating at 20-120 kV
- 0.2nm line resolution
- LaB6 emitter
- Gatan Rio CCD camera

Provides simultaneous imaging and electrophysiology, plus uncaging and photostimulation. Secondary scan path allows for simultaneous imaging and photostimulation. Point photoactivation achieved with sub-micron positioning controls.

STELLARIS 8 is a true confocal point scanning system, including a tuneable White Light Laser and a UV laser extending the detection range from 405 to near InfraRed (850). The system comes with 4 highly sensitive prism-based spectral detectors with computer controlled adjustable emission bandwidth. Lightning technology provides super resolution imaging down to 120nm resolution in xy. Both Falcon and TauSense technology provide a set of tools based on fluorescence lifetime imaging microscopy (FLIM) for improved image quality and separation of spectrally overlapping fluorophores. The system has high resolution and high depth objectives to accommodate a wide range of research needs.
Features include:
- High-end HpZ6G4 workstation with CUDA support for high workloads
- LAS X Stellaris Software for live data mode, c3D visualization, olocalization, FRAT, FRET, and electrophysiology
- LAS X Lightning Expert is advanced software for confocal, STED, and multiphoton data
- LAS C Co-Localization
- Power HyD S detector – high performance
- Power Hybrid Detector HyD X- specialized for FALCON (Fast Lifetime CONtrast)
- Tuneable lasers up to 850nm
- Objectives – 5x, 10x, 20x, 25x, 40x, 63x
- Additional workstation with full software suite for offline data analysis

The Olympus FV1200 2-Photon Microscope provides multiphoton imaging from the blue to far red range. With a 25X objective, you can achieve a wide field of view with high optical resolution. This sytem is set up for easy acquision of seconnd harmonics.
Rate Schedule (internal / external)
- Technician rate (per hour): $70 / $115
- Bruker Coherent 2-Photon Microscope (per hour): $40 / $100
- FEI TecnaiSpirit TEM (per hour): $40 / $350
- Nikon SoRa Spinning Disk Confocal Microscope (per hour): $40 / $100
- Olympus 2-Photon Microscope (per hour): $40 / $100
- Stellaris 8 Confocal Microscope (per hour): $40 / $100
- ThermoFisher Apreo VolumeScope Block Face SEM (per hour): $40 / $200
- Zeiss 700 Confocal Microscope (per hour): $15 / $100
- Training is required for each instrument.
- Users must begin by contacting facility director Christie Lacy, (christie21@vtc.vt.edu).
- Training is scheduled and provided one-on-one by the facility director.
- Each user must complete at least two training sessions.
- The intial training session typically takes 2-3 hours and covers basic operating of the instrument.
- The second session is for the user to practice with their own sample.
- Any subsequent training is based on the needs of the user.
To initiate use of cellular and molecular imaging equipment:
- Contact facility director Christie Lacy, (christie21@vtc.vt.edu).
- Complete required equipment training with facility manager.
- Access to the scheduling tool is granted upon completing of training.Users may schedule 3-hour time blocks each day on an instrument.
- Users must be trained on an instrument to schedule use of it.
- Currently some instruments have other restrictions on the amount of time that can be reserved.
- Two weeks prior to the day of a reserveration, users may extend their block past three hours if time on the instrument is available.
- Users more than 15 minutes late to a reserved time block may lose their time to another user.
- Users must clean up after themselves.
- Only leave the instrument on for the next user IF they are present have asked you to do so.
Acknowledgement and Co-authorship Guidance
Proper acknowledgement is essential for the continued funding and growth of the Cell and Molecular Imaging Core (CMIC). All work performed with any and all CMIC instruments should be acknowledged in scholarly reports, presentations, posters, papers, and all other publications. Our staff are scientist who depend on acknowledgement and authorship (when appropriate) to continue their careers. Please see the guidance below for acknowledgement and authorship.
Mandatory Minimum - Independent use of CMIC resources
“The authors acknowledge resources and support from the Cellular and Molecular Imaging Core, part of the Fralin Biomedical Research Institute at VTC. This research would not be possible without the use of the Nikon Super Resolution Spinning Disk Microscope.”
Example 1 - Independent use of CMIC resources and guidance from staff
“The authors acknowledge resources and support from the Cellular and Molecular Imaging Core, part of the Fralin Biomedical Research Institute at VTC. This research would not be possible without the use of the Leica Stellaris 8 and staff name for helpful insight in sample preparation and instrument operation.”
Example 2 - Routine data collected by CMIC personnel (data interpreted by the user)
“The authors acknowledge resources and support from the Cellular and Molecular Imaging Core, part of the Fralin Biomedical Research Institute at VTC. This research would not be possible without the use of the Thermo Fisher Aprea 2 Serial Blockface Scanning Electron Microscope (NIH award 1S10OD026838-01A1) and staff name for collecting the data shown in Figure Y."
Please always notify the relevant Facility when acknowledgment is made.
If we did more than above – please consider authorship.
Grant Proposal Information
The Cellular and Molecular Imaging Core, part of the Fralin Biomedical Research Institute at VTC, provides expertise and instrumentation for conventional transmission, serial blockface scanning electron microscopy, high resolution light microscopy, sample preparation, and immunogold labeling. Instrumentation includes: Serial Blockface Scanning Electron Microscope: ThermoFisher Aprea 2 (NIH award 1S10OD026838-01A1), Transmission Electron Microscope: FEI T12 Technai Spirit BT, Light microscopes: Nikon Super Resolution Spinning Disk Confocal, Leica Stellaris 8, Zeiss 700 Confocal microscope, and Bruker Ultima In Vitro 2-Photon Microscope.
MRI/PET
- Internal Rate O&M 230994-12990: $250
- Internal Total (Banner Funds): $250
- External Rate O&M 230994-12997: $250
- External Rate OH 230994-12920: $150
- External Total (Bursar’s Office): $400
MRI/PET Extended Run
- Internal Rate O&M 230994-12990: $150
- Internal Total (Banner Funds): $150
- External Rate O&M 230994-12997: $150
- External Rate OH 230994-12920: $90
- External Total (Bursar’s Office): $240
MRI/PET Contrast ml
- Internal Rate O&M 230994-12990: $57
- Internal Total (Banner Funds): $57
- External Rate O&M 230994-12997: $57
- External Rate OH 230994-12920: $34.20
- External Total (Bursar’s Office): $91.20
Micro PET/CT
- Internal Rate O&M 230994-12990: $250
- Internal Total (Banner Funds): $250
- External Rate O&M 230994-12997: $250
- External Rate OH 230994-12920: $150
- External Total (Bursar’s Office): $400
Micro PET/CT Tracer mCi
- Internal Rate O&M 230994-12990: $31
- Internal Total (Banner Funds): $31
- External Rate O&M 230994-12997: $31
- External Rate OH 230994-12920: $18.60
- External Total (Bursar’s Office): $49.60
All Cellular and Molecular Imaging equipment is located at the Fralin Biomedical Research Institute at VTC in Roanoke, Virginia.
Thermo Fisher Scientific Apreo Volumescope Scanning Serial Block Face Electron Microscope: Room 2306A, 4 Riverside Circle
Nikon SoRa Inverted Spinning Disk Confocal Microscope: Room 1306, 4 Riverside Circle
Zeiss 700 Confocal Microscope: Room R1028, 2 Riverside Circle
Transmission Electron Microscope with Cryo Capacity: Room R2029, 2 Riverside Circle
Bruker Ultima in vitro Multiphoton Microscope: Room R1015, 2 Riverside Circle
Leica STELLARIS 8 Spectral Confocal Microscope: Room R3024, 2 Riverside Circle
Olympus 2-photon Microscope: Room R1028, 2 Riverside Circle
Parking: During business hours, visitors may park in designated visitor parking places at the entrance to the parking garage at 2 Riverside Circle. Visitors may also park on the 2nd - 6th floors of the parking garage located at 5 Riverside Circle. On nights and weekends, visitors may park anywhere in the 5 Riverside parking garage, as well as the surface lot and parking deck. All parking is free.


