Pan Lab
-
Home Item
 Yuchin Albert Pan, Ph.D. , home
Yuchin Albert Pan, Ph.D. , homeAssociate Professor and Commonwealth Research Commercialization Fund Eminent Research Scholar in Developmental Neuroscience
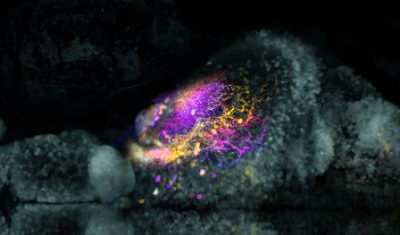
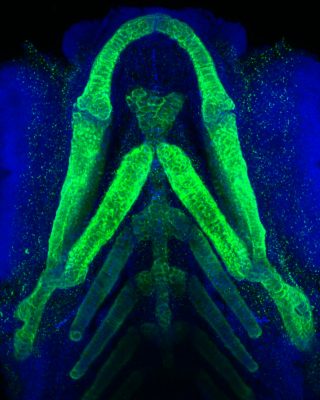
Led by principal investigator Yuchin Albert Pan, Ph.D., the Pan Lab uses zebrafish to uncover the general principles of vertebrate nervous system development. Using various zebrafish mutants and disease models, the Pan lab aims to gain fundamental insights about how abnormal neural development may contribute to human neuropsychiatric disorders.
The immense structural complexity of the human brain, which contains ~80 billion neurons and trillions of synapses, makes it challenging to parse out how developmental mechanisms affect brain function. Zebrafish serves as a more accessible vertebrate model to explore this problem, as its brain is smaller (~100,000 neurons in the larvae) and optically translucent. Furthermore, more than 70 percent of human disease genes have functional homologs in zebrafish, making it possible to generate disease-specific zebrafish models to investigate the pathophysiology of human disorders. In recent studies, we have used genome-targeting techniques (TALEN and CRISPR) to generate zebrafish mutants that elucidate the neurological and molecular causes of human diseases.
- Ates KM, Wang T, Moreland T, Veeranan-Karmegam R, Ma M, Jeter C, Anand P, Wenzel W, Kim HG, Wolfe LA, Stephen J, Adams DR, Markello T, Tifft CJ, Settlage R, Gahl WA, Gonsalvez GB, Malicdan MC, Flanagan-Steet H, Pan YA. (2020). Deficiency in the endocytic adaptor proteins PHETA1/2 impairs renal and craniofacial development. Disease Models & Mechanisms.
- Ma M, Ramirez AD, Wang T, Roberts RL, Harmon KE, Schoppik D, Sharma A, Kuang C, Goei SL, Gagnon JA, Zimmerman S, Tsai SQ, Reyon D, Joung JK, Aksay ERF, Schier AF, Pan YA. (2020). Zebrafish dscaml1 Deficiency Impairs Retinal Patterning and Oculomotor Function. Journal of Neuroscience.
We have a long-standing interest in developing new tools and reagents for visualizing anatomy, development, and neuronal connectivity (e.g. Brainbow and transsynaptic neural circuit tracers). We have developed viral neural circuit tracing tools that combine cell-type-specific targeting, axonal tracing, and physiological analyses. The application of these tools will allow rapid and systematic mapping of the zebrafish brain and provide an entry point to understand how genes affect connectivity in neuropsychiatric disorders.
- Ma M, Kler S, Pan YA. (2020). Structural Neural Connectivity Analysis in Zebrafish With Restricted Anterograde Transneuronal Viral Labeling and Quantitative Brain Mapping. Frontiers In Neural Circuits.
The brain rapidly adapts to external and internal changes, or stressors, to avoid potential harm and maintain homeostasis. One of the central pathways controlling stress response is the hypothalamus-pituitary-adrenal (HPA) axis. Dysregulation of the HPA axis has been linked to stress-associated disorders such as anxiety and depression. We are interested in how intercellular communication between neurons and glial cells controls the development of the HPA axis and identify the molecular mediators that may be targeted to alter the resilience and susceptibility of the HPA axis to early-life stressors.
-
Bio Item
 Michael Brooks, D.V.M. , bio
Michael Brooks, D.V.M. , bioGraduate Student, Department Biomedical Sciences and Pathobiology, Virginia-Maryland College of Veterinary Medicine
-
Bio Item
 Maryam Iqbal , bio
Maryam Iqbal , bioMedical Student, Virginia Tech Carilion School of Medicine '27
-
Bio Item
 Ranya Ridha , bio
Ranya Ridha , bioResearch Assistant
-
Bio Item
 Stephen Stoyanof , bio
Stephen Stoyanof , bioResearch Associate