Smyth Lab
-
Home Item
 James Smyth, Ph.D. , home
James Smyth, Ph.D. , homeAssociate Professor
The Smyth Laboratory, led by James Smyth, Ph.D., investigates how stressors, including ischemia and viral infection, disrupt intercellular communication in the heart, creating arrhythmogenic substrates. The lab's work focuses on gap junctions formed by connexin 43 (Cx43), the principal isoform in ventricular myocardium, which electrically couple cardiomyocytes to enable synchronized contraction. In essentially all forms of cardiomyopathy, loss or altered regulation of Cx43 occurs and results in impaired electrical conduction and contributes to sudden cardiac death. The team's over-arching goal is to identify critical therapeutic targets to restore or preserve normal electrical coupling in diseased hearts and prevent the arrhythmias of sudden cardiac death.
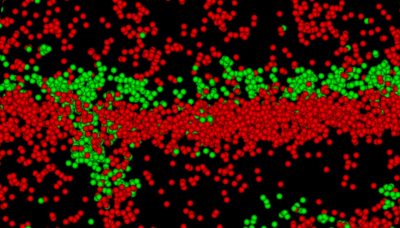
Specifically, the lab investigates how regulation of mRNA translation, particularly alternative ‘internal’ translation initiation, generates N-terminally truncated Cx43 isoforms that influence gap junction trafficking and assembly. These alternative translational events are dynamically controlled by stress-responsive signaling pathways, including during cardiac ischemia. Such molecular insights allow the team to test novel interventions that enhance gap junction integrity under stress and prevent arrhythmogenic remodeling at the subcellular level. The lab is also extending this work to cancer, where internally translated Cx43 isoforms and non-channel functions of connexins contribute to altered cell signaling, invasive behavior, and therapeutic resistance.
As molecular virologists, the lab team studies in parallel how respiratory viruses such as adenovirus and coronavirus target the host translational machinery and gap junction network to enhance their replication and spread. Adenovirus is a leading cause of viral myocarditis but species specificity has historically limited research in this area. Through implementation of human induced pluripotent stem cell technologies and novel models the lab has identified rapid targeting of gap junction function during adenoviral infection in epithelial and cardiac cells. This work has revealed how acute viral infection disrupts electrical conduction prior to inflammation, immune infiltration, or overt cardiomyopathy; uncovering a previously understudied and arrhythmogenic ‘pre-myocarditis’ stage of infection. The team is investigating specifically how viruses reprogram the cell at the molecular level to harness this information therapeutically for myocarditis and beyond.
Using a combination of RNA biology, biochemical assays, live-cell imaging, and super-resolution microscopy, the Smyth Lab aims to uncover new therapeutic strategies to restore electrical integrity in the injured and infected heart.
-
Bio Item
 Chelsea Phillips, Ph.D. , bio
Chelsea Phillips, Ph.D. , bioPostdoctoral Associate
-
Bio Item
 Veronica Qu , bio
Veronica Qu , bioMedical Student, Virginia Tech Carilion School of Medicine '27
-
Bio Item
 Eric Sapp , bio
Eric Sapp , bioResearch Associate, BSL3 Core Manager
-
Bio Item
 Evangeline Scheibe , bio
Evangeline Scheibe , bioMedical Student, Virginia Tech Carilion School of Medicine '27
-
Bio Item
 Kenneth Young , bio
Kenneth Young , bioMedical Student, Virginia Tech Carilion School of Medicine '26
-
Bio Item
 Michael Zeitz, Ph.D. , bio
Michael Zeitz, Ph.D. , bioResearch Assistant Professor