Exercise-induced mitophagy
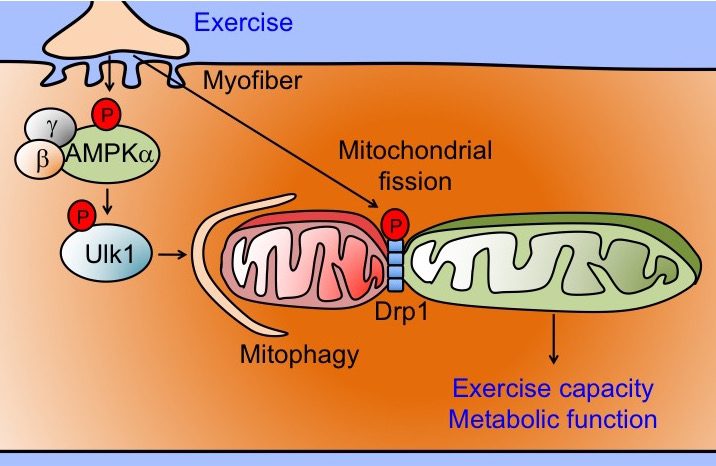
Non-communicable diseases, killing 38 million people worldwide each year, are often caused/exacerbated by accumulation of damaged/dysfunctional mitochondria. It is well-known that egular exercise improves mitochondrial function and is considered the most powerful intervention for the prevention of non-communicable diseases; however, the underlying molecular and cellular mechanisms remain largely unknown, hindering our ability to optimize exercise intervention and develop more effective therapeutics. Autophagy, a conserved cellular degradation process for aggregated proteins and damaged organelles, is known to be activated in skeletal muscle by exercise; however, the regulation and functional role of mitophagy, a specific autophagic clearance process for mitochondria, in skeletal muscle by exercise training is poorly understood. Using a novel mitochondrial reporter gene, pMitoTimer, for quantification of mitochondrial oxidative stress and mitophagy in vivo, the Yan Lab has shown that a single bout of endurance exercise induces mitochondrial oxidative stress and mitophagy in skeletal muscle, current/preceded by activation of the nutrient/energy sensor 5’ AMP-activated protein kinase (AMPK), autophagy protein unc-51 like autophagy activating kinase 1 (Ulk1) and mitochondrial fission mediator dynamin-related protein 1 (Drp1). The lab's current research focuses on the role of exercise-induced activation of AMPK and Ulk1 along with Drp1-mediated mitochondrial fission in the regulation of mitophagy in promoting mitochondrial quality, and contractile and metabolic adaptations.
Publications
Yan Z, Lira VA, Greene NP. Exercise training-induced regulation of mitochondrial quality. Exerc Sport Sci Rev. 2012 Jul;40(3):159-64. doi: 10.1097/JES.0b013e3182575599. Review. PubMed PMID: 22732425; PubMed Central PMCID: PMC3384482.
Lira VA, Okutsu M, Zhang M, Greene NP, Laker RC, Breen DS, Hoehn KL, Yan Z. Autophagy is required for exercise training-induced skeletal muscle adaptation and improvement of physical performance. FASEB J. 2013 Oct;27(10):4184-93. doi: 10.1096/fj.13-228486. Epub 2013 Jun 27. PubMed PMID: 23825228; PubMed Central PMCID: PMC4046188.
Laker RC, Xu P, Ryall KA, Sujkowski A, Kenwood BM, Chain KH, Zhang M, Royal MA, Hoehn KL, Driscoll M, Adler PN, Wessells RJ, Saucerman JJ, Yan Z. A novel MitoTimer reporter gene for mitochondrial content, structure, stress, and damage in vivo. J Biol Chem. 2014 Apr 25;289(17):12005-15. doi: 10.1074/jbc.M113.530527. Epub 2014 Mar 18. PubMed PMID: 24644293; PubMed Central PMCID: PMC4002107.
Booth FW, Ruegsegger GN, Toedebusch RG, Yan Z. Endurance Exercise and the Regulation of Skeletal Muscle Metabolism. Prog Mol Biol Transl Sci. 2015;135:129-51. doi: 10.1016/bs.pmbts.2015.07.016. Epub 2015 Sep 5. Review. PubMed PMID: 26477913.
Drake JC, Wilson RJ, Yan Z. Molecular mechanisms for mitochondrial adaptation to exercise training in skeletal muscle. FASEB J. 2016 Jan;30(1):13-22. doi: 10.1096/fj.15-276337. Epub 2015 Sep 14. Review. PubMed PMID: 26370848; PubMed Central PMCID: PMC6137621.
Leitner LM, Wilson RJ, Yan Z, Gödecke A. Reactive Oxygen Species/Nitric Oxide Mediated Inter-Organ Communication in Skeletal Muscle Wasting Diseases. Antioxid Redox Signal. 2017 May 1;26(13):700-717. doi: 10.1089/ars.2016.6942. Epub 2017 Jan 4. Review. PubMed PMID: 27835923; PubMed Central PMCID: PMC5421600.
Call JA, Wilson RJ, Laker RC, Zhang M, Kundu M, Yan Z. Ulk1-mediated autophagy plays an essential role in mitochondrial remodeling and functional regeneration of skeletal muscle. Am J Physiol Cell Physiol. 2017 Jun 1;312(6):C724-C732. doi: 10.1152/ajpcell.00348.2016. Epub 2017 Mar 29. PubMed PMID: 28356270; PubMed Central PMCID: PMC5494591.
Yan Z, Kronemberger A, Blomme J, Call JA, Caster HM, Pereira RO, Zhao H, de Melo VU, Laker RC, Zhang M, Lira VA. Exercise leads to unfavourable cardiac remodelling and enhanced metabolic homeostasis in obese mice with cardiac and skeletal muscle autophagy deficiency. Sci Rep. 2017 Aug 11;7(1):7894. doi: 10.1038/s41598-017-08480-2. PubMed PMID: 28801668; PubMed Central PMCID: PMC5554260.
Laker RC, Drake JC, Wilson RJ, Lira VA, Lewellen BM, Ryall KA, Fisher CC, Zhang M, Saucerman JJ, Goodyear LJ, Kundu M, Yan Z. Ampk phosphorylation of Ulk1 is required for targeting of mitochondria to lysosomes in exercise-induced mitophagy. Nat Commun. 2017 Sep 15;8(1):548. doi: 10.1038/s41467-017-00520-9. PubMed PMID: 28916822; PubMed Central PMCID: PMC5601463.
Drake JC, Yan Z. Mitophagy in maintaining skeletal muscle mitochondrial proteostasis and metabolic health with ageing. J Physiol. 2017 Oct 15;595(20):6391-6399. doi: 10.1113/JP274337. Epub 2017 Sep 24. Review. PubMed PMID: 28795394; PubMed Central PMCID: PMC5638883.
Wilson RJ, Drake JC, Cui D, Zhang M, Perry HM, Kashatus JA, Kusminski CM, Scherer PE, Kashatus DF, Okusa MD, Yan Z. Conditional MitoTimer reporter mice for assessment of mitochondrial structure, oxidative stress, and mitophagy. Mitochondrion. 2019 Jan;44:20-26. doi: 10.1016/j.mito.2017.12.008. Epub 2017 Dec 20. PubMed PMID: 29274400.
Drake JC, Laker RC, Wilson RJ, Zhang M, Yan Z. Exercise-induced mitophagy in skeletal muscle occurs in the absence of stabilization of Pink1 on mitochondria. Cell Cycle. 2018 Dec 17. doi: 10.1080/15384101.2018.1559556. [Epub ahead of print] PubMed PMID: 30558471.
Xu P, Damschroder D, Zhang M, Ryall KA, Adler PN, Saucerman JJ, Wessells RJ, Yan Z. Atg2, Atg9 and Atg18 in mitochondrial integrity, cardiac function and healthspan in Drosophila. J Mol Cell Cardiol. 2018 Dec 17;127:116-124. doi: 10.1016/j.yjmcc.2018.12.006. [Epub ahead of print] PubMed PMID: 30571977.